72 Ionic Bonds and Ionic Compounds Section Review
Chemic Bonding and Molecular Geometry
38 Ionic Bonding
Learning Objectives
By the end of this department, yous will be able to:
- Explain the formation of cations, anions, and ionic compounds
- Predict the charge of mutual metallic and nonmetallic elements, and write their electron configurations
As you take learned, ions are atoms or molecules begetting an electrical charge. A cation (a positive ion) forms when a neutral atom loses ane or more electrons from its valence crush, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence vanquish.
Compounds composed of ions are called ionic compounds (or salts), and their elective ions are held together by ionic bonds: electrostatic forces of attraction between oppositely charged cations and anions. The backdrop of ionic compounds shed some lite on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they besides tend to have loftier melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are as well poor conductors of electricity for the aforementioned reason—the strength of ionic bonds prevents ions from moving freely in the solid land. Almost ionic solids, nonetheless, deliquesce readily in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely.
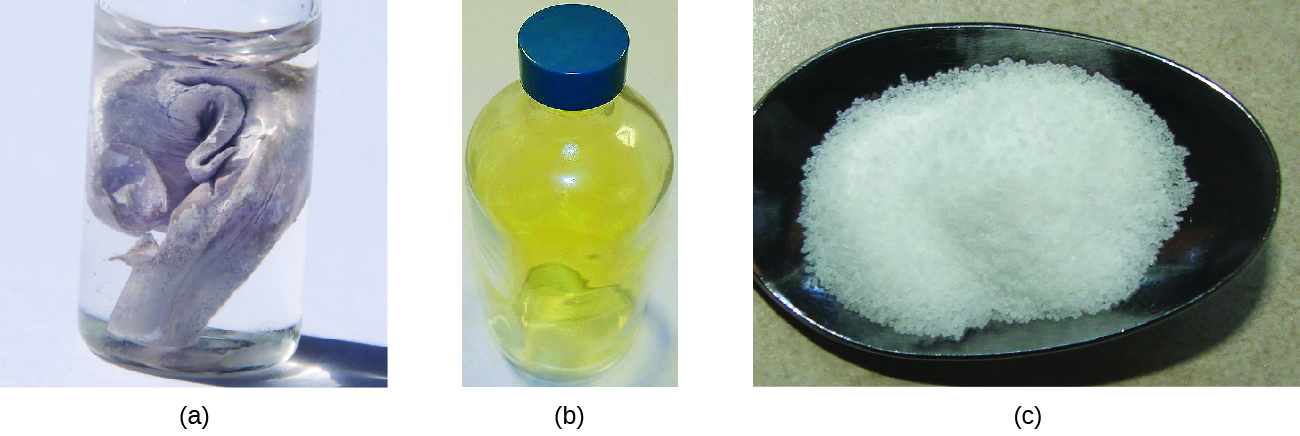
Neutral atoms and their associated ions have very dissimilar concrete and chemic properties. Sodium atoms course sodium metal, a soft, silvery-white metal that burns vigorously in air and reacts explosively with water. Chlorine atoms form chlorine gas, Cl2, a yellow-dark-green gas that is extremely corrosive to about metals and very poisonous to animals and plants. The vigorous reaction betwixt the elements sodium and chlorine forms the white, crystalline chemical compound sodium chloride, common table salt, which contains sodium cations and chloride anions ((Effigy)). The compound composed of these ions exhibits properties entirely unlike from the properties of the elements sodium and chlorine. Chlorine is poisonous, merely sodium chloride is essential to life; sodium atoms react vigorously with water, merely sodium chloride merely dissolves in water.
(a) Sodium is a soft metal that must exist stored in mineral oil to prevent reaction with air or h2o. (b) Chlorine is a pale xanthous-greenish gas. (c) When combined, they course white crystals of sodium chloride (table salt). (credit a: modification of work by "Jurii"/Wikimedia Commons)
The Germination of Ionic Compounds
Binary ionic compounds are composed of just ii elements: a metal (which forms the cations) and a nonmetal (which forms the anions). For example, NaCl is a binary ionic chemical compound. We can retrieve about the germination of such compounds in terms of the periodic properties of the elements. Many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a flow or most the lesser of a group on the periodic table. Nonmetal atoms have relatively high electron affinities and thus readily gain electrons lost by metal atoms, thereby filling their valence shells. Nonmetallic elements are plant in the upper-right corner of the periodic tabular array.
As all substances must be electrically neutral, the total number of positive charges on the cations of an ionic compound must equal the total number of negative charges on its anions. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative charges. For example, the formula for aluminum oxide, Al2O3, indicates that this ionic chemical compound contains two aluminum cations, Althree+, for every three oxide anions, O2− [thus, (two ![]() +3) + (iii
+3) + (iii ![]() –two) = 0].
–two) = 0].
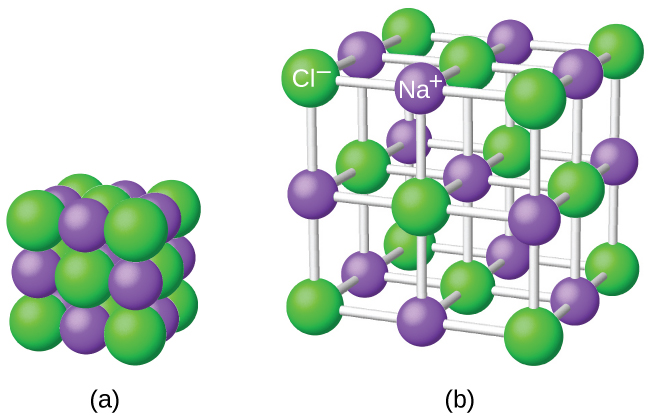
It is important to note, however, that the formula for an ionic compound does non stand for the concrete organisation of its ions. It is wrong to refer to a sodium chloride (NaCl) "molecule" because there is not a single ionic bond, per se, betwixt whatsoever specific pair of sodium and chloride ions. The attractive forces between ions are isotropic—the same in all directions—pregnant that any particular ion is equally attracted to all of the nearby ions of opposite charge. This results in the ions arranging themselves into a tightly bound, three-dimensional lattice construction. Sodium chloride, for example, consists of a regular system of equal numbers of Na+ cations and Cl– anions ((Figure)).
The atoms in sodium chloride (common table common salt) are bundled to (a) maximize contrary charges interacting. The smaller spheres represent sodium ions, the larger ones represent chloride ions. In the expanded view (b), the geometry can be seen more than conspicuously. Notation that each ion is "bonded" to all of the surrounding ions—six in this instance.
The strong electrostatic attraction between Na+ and Cl– ions holds them tightly together in solid NaCl. It requires 769 kJ of energy to dissociate 1 mole of solid NaCl into separate gaseous Na+ and Cl– ions:
![]()
Electronic Structures of Cations
When forming a cation, an cantlet of a main group element tends to lose all of its valence electrons, thus assuming the electronic structure of the noble gas that precedes it in the periodic tabular array. For groups 1 (the alkali metals) and 2 (the alkaline world metals), the group numbers are equal to the numbers of valence beat electrons and, consequently, to the charges of the cations formed from atoms of these elements when all valence shell electrons are removed. For example, calcium is a group 2 element whose neutral atoms take 20 electrons and a ground state electron configuration of is ii2s 22p 63south two3p 6foursouthward 2. When a Ca atom loses both of its valence electrons, the result is a cation with xviii electrons, a 2+ accuse, and an electron configuration of 1s 22s ii2p 6iiis two3p 6. The Ca2+ ion is therefore isoelectronic with the noble gas Ar.
For groups 13–17, the grouping numbers exceed the number of valence electrons by ten (accounting for the possibility of total d subshells in atoms of elements in the fourth and greater periods). Thus, the charge of a cation formed by the loss of all valence electrons is equal to the group number minus x. For example, aluminum (in grouping 13) forms 3+ ions (Al3+).
Exceptions to the expected behavior involve elements toward the bottom of the groups. In addition to the expected ions Tliii+, Sn4+, Pb4+, and Bi5+, a partial loss of these atoms' valence trounce electrons can too pb to the formation of Tl+, Sn2+, Pbii+, and Bi3+ ions. The formation of these 1+, 2+, and 3+ cations is ascribed to the inert pair effect, which reflects the relatively low energy of the valence due south-electron pair for atoms of the heavy elements of groups 13, 14, and 15. Mercury (group 12) also exhibits an unexpected behavior: it forms a diatomic ion, ![]() (an ion formed from 2 mercury atoms, with an Hg-Hg bail), in addition to the expected monatomic ion Hg2+ (formed from only 1 mercury cantlet).
(an ion formed from 2 mercury atoms, with an Hg-Hg bail), in addition to the expected monatomic ion Hg2+ (formed from only 1 mercury cantlet).
Transition and inner transition metal elements behave differently than main group elements. Most transition metallic cations have 2+ or 3+ charges that upshot from the loss of their outermost s electron(southward) first, sometimes followed by the loss of one or ii d electrons from the side by side-to-outermost beat out. For instance, iron (isouth two2southward 22p half-dozen3s two3p 6threed 64s 2) forms the ion Irontwo+ (anes 22s two2p six3south 2threep 6threed 6) by the loss of the 4s electron and the ion Fe3+ (is 22southward two2p 63s 23p 63d 5) by the loss of the 4s electron and ane of the iiid electrons. Although the d orbitals of the transition elements are—according to the Aufbau principle—the concluding to fill when building up electron configurations, the outermost s electrons are the beginning to be lost when these atoms ionize. When the inner transition metals form ions, they commonly have a 3+ charge, resulting from the loss of their outermost southward electrons and a d or f electron.
Determining the Electronic Structures of Cations In that location are at to the lowest degree fourteen elements categorized every bit "essential trace elements" for the homo trunk. They are chosen "essential" because they are required for healthy bodily functions, "trace" because they are required but in small amounts, and "elements" in spite of the fact that they are actually ions. Two of these essential trace elements, chromium and zinc, are required as Cr3+ and Zn2+. Write the electron configurations of these cations.
Solution First, write the electron configuration for the neutral atoms:
Zn: [Ar]iiid 10fours 2
Cr: [Ar]3d 54s 1
Side by side, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and so from the d orbital. For the p-block elements, electrons are removed from the p orbitals and then from the due south orbital. Zinc is a fellow member of group 12, so it should have a accuse of 2+, and thus loses just the two electrons in its south orbital. Chromium is a transition element and should lose its s electrons and then its d electrons when forming a cation. Thus, we find the post-obit electron configurations of the ions:
Zntwo+: [Ar]threed ten
Cr3+: [Ar]3d three
Check Your Learning Potassium and magnesium are required in our nutrition. Write the electron configurations of the ions expected from these elements.
Answer:
K+: [Ar], Mg2+: [Ne]
Electronic Structures of Anions
Nearly monatomic anions form when a neutral nonmetal atom gains plenty electrons to completely fill up its outer s and p orbitals, thereby reaching the electron configuration of the next element of group 0. Thus, information technology is elementary to determine the charge on such a negative ion: The charge is equal to the number of electrons that must be gained to fill the due south and p orbitals of the parent atom. Oxygen, for example, has the electron configuration onesouth twoiis 2twop 4, whereas the oxygen anion has the electron configuration of the noble gas neon (Ne), 1s 22due south 2iip vi. The two boosted electrons required to fill the valence orbitals requite the oxide ion the charge of 2– (Otwo–).
Determining the Electronic Structure of Anions Selenium and iodine are two essential trace elements that form anions. Write the electron configurations of the anions.
Solution Se2–: [Ar]3d 10foursouth two4p six
I–: [Kr]4d 105due south 25p 6
Check Your Learning Write the electron configurations of a phosphorus atom and its negative ion. Give the accuse on the anion.
Answer:
P: [Ne]threes 23p 3; Pthree–: [Ne]3s 23p 6
Key Concepts and Summary
Atoms gain or lose electrons to form ions with particularly stable electron configurations. The charges of cations formed past the representative metals may be determined readily because, with few exceptions, the electronic structures of these ions have either a noble gas configuration or a completely filled electron shell. The charges of anions formed past the nonmetals may also be readily determined considering these ions form when nonmetal atoms gain enough electrons to fill their valence shells.
Chemical science End of Chapter Exercises
Does a cation proceeds protons to course a positive charge or does it lose electrons?
The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Positive charges form when electrons are lost.
Atomic number 26(3) sulfate [Fe2(SO4)three] is composed of Feiii+ and ![]() ions. Explain why a sample of iron(III) sulfate is uncharged.
ions. Explain why a sample of iron(III) sulfate is uncharged.
Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: P, I, Mg, Cl, In, Cs, O, Pb, Co?
P, I, Cl, and O would form anions because they are nonmetals. Mg, In, Cs, Pb, and Co would form cations considering they are metals.
Which of the post-obit atoms would be expected to form negative ions in binary ionic compounds and which would exist expected to grade positive ions: Br, Ca, Na, North, F, Al, Sn, South, Cd?
Predict the accuse on the monatomic ions formed from the following atoms in binary ionic compounds:
(a) P
(b) Mg
(c) Al
(d) O
(east) Cl
(f) Cs
(a) P3–; (b) Mgii+; (c) Al3+; (d) O2–; (eastward) Cl–; (f) Cs+
Predict the accuse on the monatomic ions formed from the following atoms in binary ionic compounds:
(a) I
(b) Sr
(c) 1000
(d) N
(e) S
(f) In
Write the electron configuration for each of the following ions:
(a) Equally3–
(b) I–
(c) Be2+
(d) Cdii+
(e) O2–
(f) Ga3+
(m) Li+
(h) Niii–
(i) Sn2+
(j) Co2+
(k) Feii+
(l) Asthree+
(a) [Ar]4s 23d 104p 6; (b) [Kr]fourd ten5south 25p 6 (c) isouth 2 (d) [Kr]4d 10; (e) [He]2s two2p half-dozen; (f) [Ar]iiid 10; (g) 1southward two (h) [He]iis iitwop 6 (i) [Kr]4d 10fives two (j) [Ar]3d 7 (k) [Ar]3d 6, (l) [Ar]threed tenivsouth ii
Write the electron configuration for the monatomic ions formed from the following elements (which form the greatest concentration of monatomic ions in seawater):
(a) Cl
(b) Na
(c) Mg
(d) Ca
(eastward) K
(f) Br
(g) Sr
(h) F
Write out the full electron configuration for each of the following atoms and for the monatomic ion constitute in binary ionic compounds containing the chemical element:
(a) Al
(b) Br
(c) Sr
(d) Li
(e) As
(f) S
(a) 1southward 22southward 22p 6threedue south 2iiip 1; Aliii+: 1south 2twosouthward iitwop half dozen; (b) isouthward iiiis twoiip half-dozen3s ii3p six3d ten4due south 2ivp 5; ones 22s ii2p half dozen3s 23p half dozen3d x4s 24p six; (c) 1s twoiisouthward 22p 6iiis twothreep 6threed 104southward 2fourp 65due south 2; Srtwo+: 1due south 22s 22p half dozenthreedue south iiiiip 63d xfours 24p six; (d) 1s 22s 1; Li+: 1due south ii; (e) 1s 22due south 2iip 63s 23p 63d 10ivs 24p 3; 1s 22s 22p 63s 2iiip half-dozen3d 104s 24p half-dozen; (f) 1s 22southward 22p six3s two3p four; is 22s 2twop 63s ii3p 6
From the labels of several commercial products, gear up a listing of six ionic compounds in the products. For each compound, write the formula. (You may demand to look up some formulas in a suitable reference.)
Glossary
- inert pair consequence
- tendency of heavy atoms to form ions in which their valence southward electrons are not lost
- ionic bond
- strong electrostatic force of attraction between cations and anions in an ionic compound
gallowaykneve1986.blogspot.com
Source: https://opentextbc.ca/chemistry2eopenstax/chapter/ionic-bonding/
0 Response to "72 Ionic Bonds and Ionic Compounds Section Review"
Post a Comment